Why Choose Phlexglobal's Clinical Services?
FREE UP YOUR INTERNAL TMF RESOURCES
Leverage seasoned Trial Master File (TMF) Study Owners anytime anywhere. Obtain pragmatic training on TMF best practices & technology. Conduct efficient, accurate TMF migrations & imports at any scale.
OPTIMIZE PROCESSES & QUALITY
Get a holistic view of completeness, timeliness & quality. Pinpoint & correct TMF completeness gaps. Embed TMF best practices around quality control & compliance. Obtain expert guidance for SOPs & eTMF technologies.
MAKE YOUR eTMF SYSTEM WORK FOR YOU
Align TMF technology with your people & processes. Leverage your eTMF to develop & automate better TMF workflows. Obtain hands-on, system-specific eTMF training. Identify what eTMF technologies can - and can’t - do well.
HOW TO SURVIVE A TMF INSPECTION WHEN YOU'RE NOT PREPARED
You’ve just received an inspection notice, and the adrenaline is starting to kick in. When was the last completeness report? Quality review? Did the team finish the latest training on SOPs and the new eTMF system? Where to start?
Help is here. Download “Don’t Panic: A TMF Inspection Guide for the Unprepared.”

TMF Completeness Heatmaps
Easily identify potential TMF problem areas in any eTMF system and enable a risk-based remediation approach.

TMF Quality Review
A thorough quality assessment of your TMF, giving you detailed insight into its quality and completeness.

TMF Document Processing
Ensure completeness, accuracy, and consistency from source to archive - regardless of your eTMF provider.

TMF Study Owners
Our TMF Practitioners have successfully mastered the challenges you and your teams face every day.

TMF Consulting Services
Our expert TMF consultants help your company reach and stay in the TMF Health Zone.

M&A Support
Get a true picture of your acquired drug’s readiness for inspection or submission - and fix any problems.

Migrations & Imports
Help ensure you are meeting GCP standards and regulatory requirements for essential documents.

TMF Expert Training
Generate lasting benefits to your team and improve the overall health of your TMF.

Veeva Vault Support
Need help with Veeva Vault? Bring Phlexglobal expertise to Vault or any other eTMF tool.
PHLEXGLOBAL EXPERTS FILL THE GAPS IN eTMF MANAGEMENT
Download our latest infographic to see how Phlexglobal’s unmatched expertise working in any eTMF system is enabling busy study teams to assess, prevent, and manage risk across the entire TMF spectrum – improvng inspection-readiness with less effort.
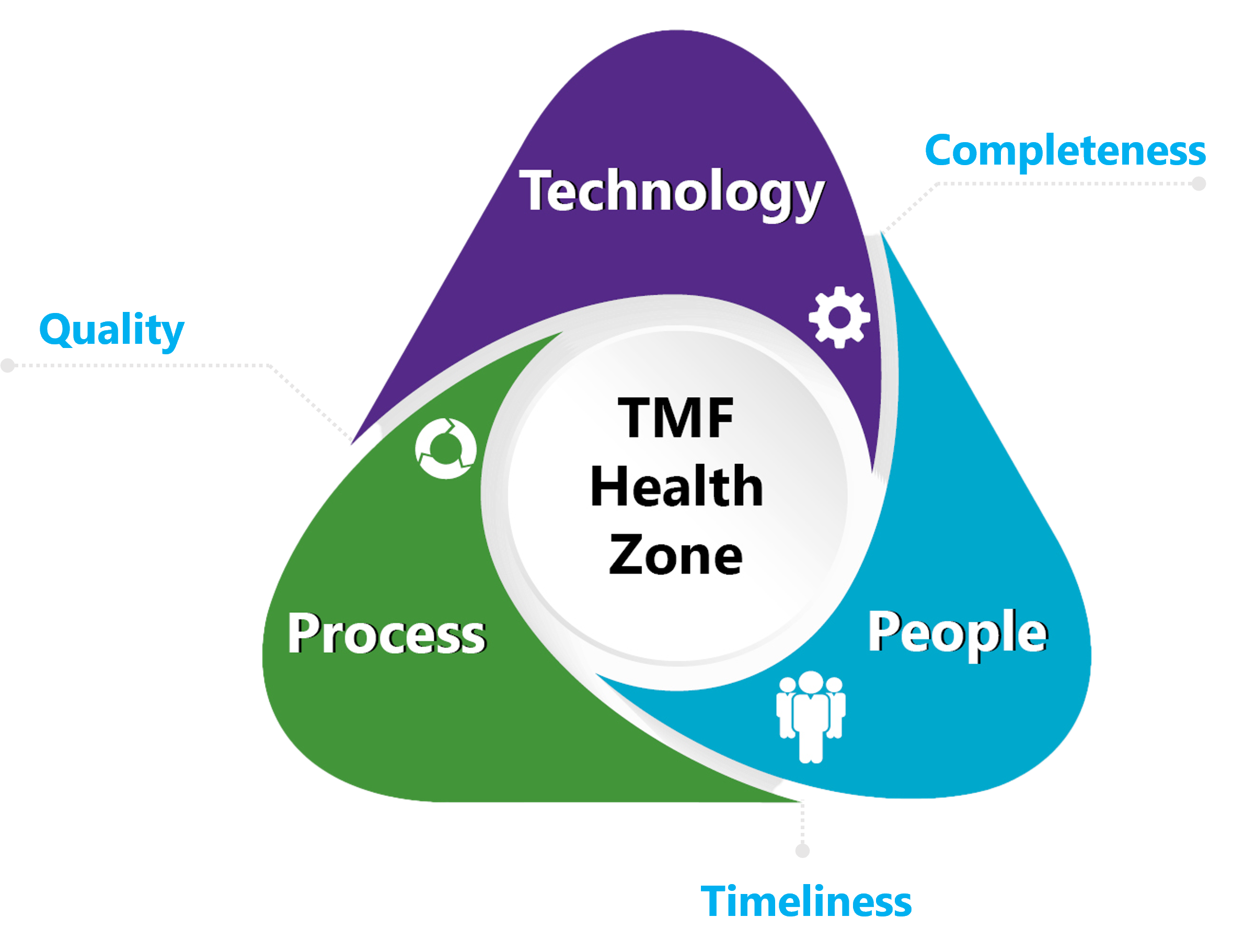
Are you in the TMF Health Zone?
For inspection-readiness, the critical attributes of TMF health are completeness, timeliness, and quality. To achieve and maintain ongoing TMF health and inspection readiness – the “TMF Health Zone” – requires experienced people trained on effective processes using the right technology.
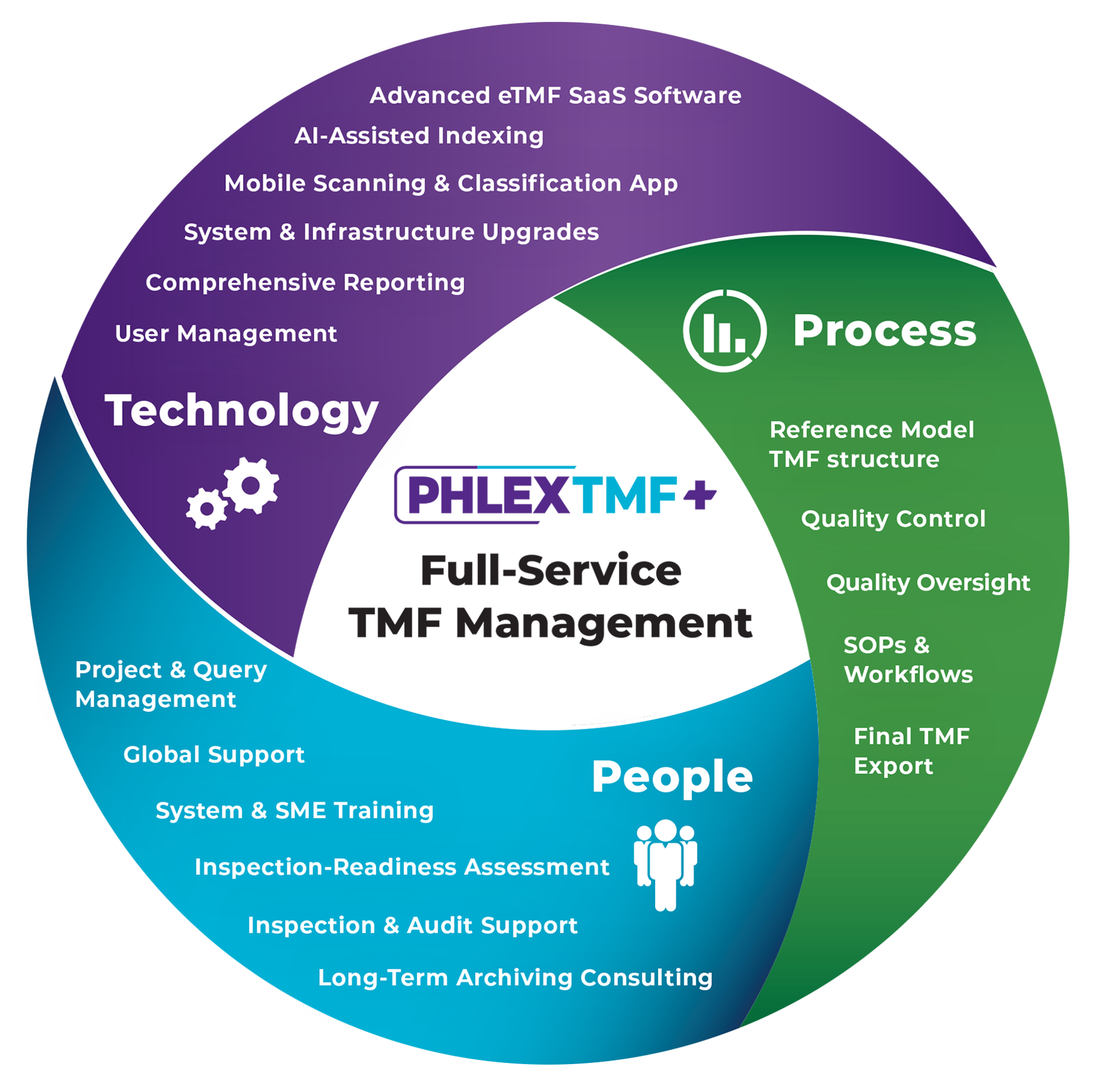
Full-Service Trial Master File Management
The Industry’s Only Turnkey, End-to-End TMF Outsourcing Solution from a Single Expert Provider.
Latest Insights

Smart Steps to Managing Your TMF Audit Trail
The term audit trail can be daunting for companies, but this is a regulatory requirement. Simply put, when it comes to the trial master file (TMF), if an action is not recorded in the audit trail,

Lowering the TMF temperature through quality reviews
We are thrilled to announce Season 2 of our exciting webinar program, Summer Shorts: TMF Excellence Edition! Avoid boring summer reruns and attend these fresh, informative sessions where our experts

Five-step Game Plan for a TMF Close-out
Close-out of a clinical trial raises many questions about responsibility and management of the trial master file (TMF). According to ICH E6 (R2), final close-out can only occur when the monitor – or
.png)
