
Phlexglobal, a PharmaLex company and the recognized global authority in Trial Master File services and technology, today announced the general availability of PhlexTMF v21: the industry’s only purpose-built eTMF solution with next-generation artificial intelligence that helps ensure clinical documents are “right-first-time” at the critical document upload step
Read more.
We are pleased to share that Trial Master File Reference Model Group has officially affiliated with CDISC. Read More

PharmaLex Group, a leading provider of specialized services for the pharma, biotech and medtech industries worldwide, has announced the merger with leading technology and services organization for clinical and regulatory solutions, Phlexglobal.

Smithson will provide proven leadership for rapidly expanding regulatory operations team supporting Phlexglobal customers worldwide.
.png?width=829&height=182&name=Phlex%20RIM%20Logo%20(transparent).png)
New PhlexRIM 2.0 provides end-to-end regulatory information management, preconfigured automations built around regulatory best practices, and flexibility to map easily to internal processes
.png?width=864&height=178&name=Phlex%20TMF%20Logo%20(transparent).png)
Renowned institution providing groundbreaking research for Covid-19 and other critical health needs required rapid transformation from paper-based processes to streamline TMF management

Companies significantly increasing speed and accuracy of document processing and quality control with PhlexTMF’s AI-assisted indexing solution trained on millions of Trial Master File documents.

Phlexglobal announced today that an increasing number of pharmaceutical companies, including five of the global Top 10, are utilizing Phlexglobal’s innovative TMF Quality Review solution to identify and mitigate potential regulatory risk for mergers & acquisitions and improve inspection readiness.

Phlexglobal announced today that Treximo, a leading life sciences consulting company and part of The Planet Group, has selected Phlexglobal and its innovative regulatory SaaS software, PhlexSubmission, as the key technology to meet growing customer needs for regulatory submissions.

Long-time TMF Reference Model supporter Phlexglobal endorses newest version offering industry-driven updates to improve the quality, consistency, and flexibility of Trial Master File management.

Fit-for-purpose eTMF SaaS software and expert services strengthens PRA customers’ confidence that their Trial Master File is compliant and inspection-ready from plan to archive
.png?width=1043&height=589&name=Covid-19%20(2).png)
Innovative respiratory disease company joins more than 15 customers drawing on Phlexglobal’s support to help develop promising COVID-19 therapies and vaccines.
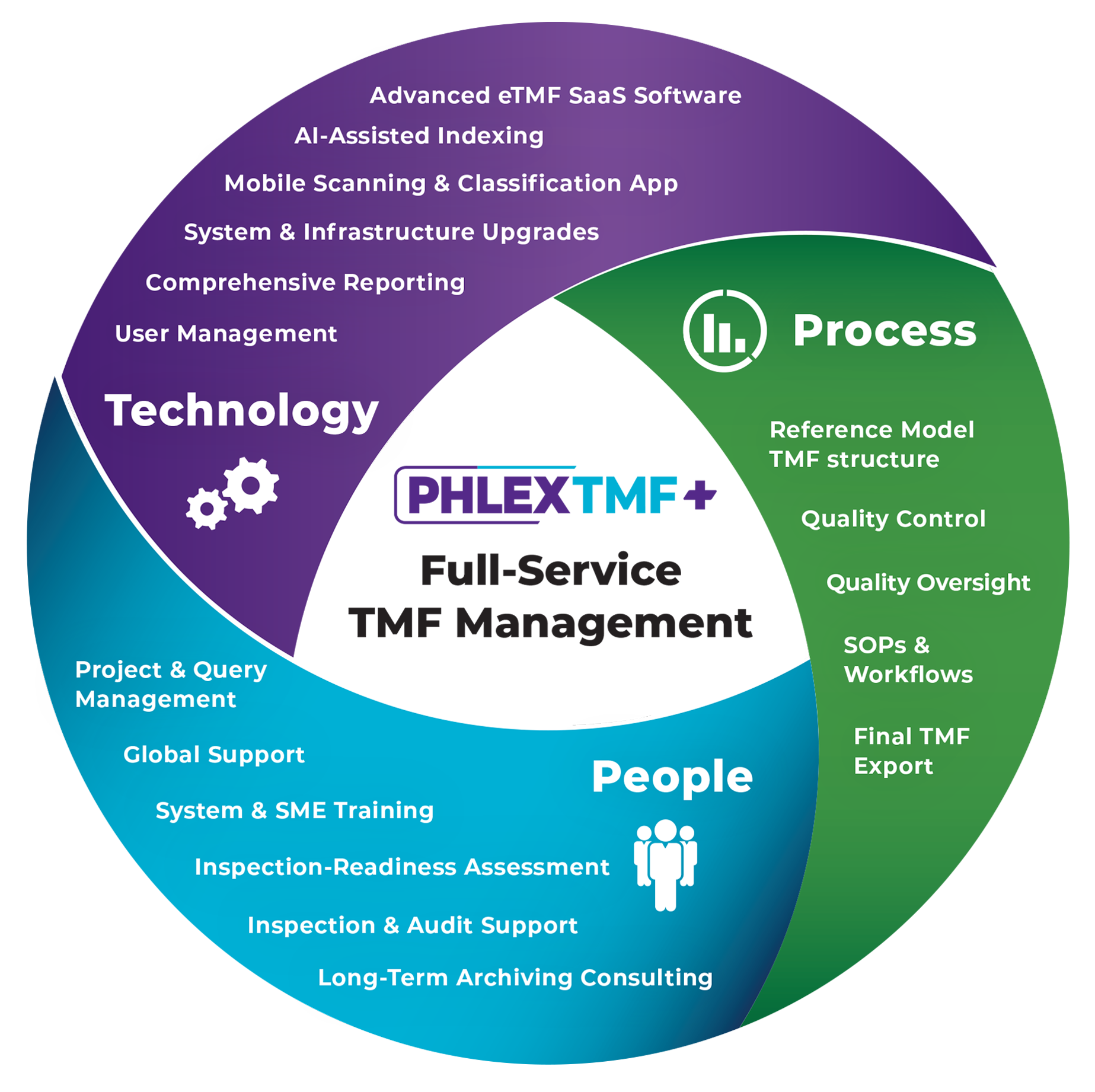
PhlexTMF+ provides a one-stop, full-service solution that encompasses all the software and services required for effective TMF management and ongoing inspection-readiness. Organizations can be confident that their Trial Master File is being managed by experts every step of the way, from planning and document processing and study closeout.
A newly announced technical partnership between Arkivum, recognised internationally for its cross-sector expertise in archiving and digital preservation, and Phlexglobal, a leading provider of clinical and regulatory software and services for the life sciences industry, will enable Phlexglobal’s customers to achieve and maintain compliance over the entire Trial Master File (TMF) lifecycle, covering both the live trial and the statutory upcoming 25-year archiving period – or longer - as required.

Phlexglobal customers, partners, and subject matter experts will share proven innovation and pragmatic guidance to help life sciences companies improve end-to-end regulatory compliance
.png?width=1043&height=589&name=Covid-19%20(2).png)
Phlexglobal, a leading innovative technology and services provider for the life sciences industry, today announces its substantial contribution to COVID-19 clinical trial activities. Since the outbreak of the COVID-19 pandemic, Phlexglobal has provided extensive support for multiple pharmaceutical companies developing effective treatments for those who have been impacted by the virus and vaccines to prevent its spread. These services are vital to developing life-saving treatments for the most important health crisis facing the world today.

Phlexglobal, the leading provider of Trial Master File (TMF) software and expert services, today announced the acquisition of Cunesoft, a move that expands the company’s portfolio of best-of-breed technologies designed to provide solution-specific functionality to meet the business objectives of life sciences companies.

Oracle Health Sciences, a leader in eClinical technology and Phlexglobal, pioneers in the provision of Trial Master File (TMF) technology and services for the global life sciences industry, have announced enhanced integrations to accelerate the speed and accuracy of regulatory compliance and inspection readiness in clinical trials.

This Autumn Phlexglobal will host the annual TMF World Forum, two exciting events focused on helping the life science industry, including pharmaceutical companies, clinical research organizations, and medical device manufacturers, meet compliance standards, achieve ongoing TMF Health, and become consistently inspection-ready: what the company refers to as “Reaching the TMF Health Zone.”

Phlexglobal today announced an exciting series of complimentary seminars aimed at helping pharmaceutical companies meet regulatory guidelines and achieve optimum TMF Health.

Phlexglobal today announced an important industry advisory around the new European Medicines Agency (EMA) guidance on Trial Master Files. The guidance took effect on June 6, 2019, and promises to have far-reaching impacts on an organization’s TMF processes, technology, and best practices – as well as related departments and systems.

Phlexglobal today announced that its Board of Directors has appointed John McNeill as Chief Executive Officer and member of the Board of Directors effective January 14, 2019. McNeill previously held the position of President at Sparta Systems, Inc.

Phlexglobal, a specialist provider of technology-enabled Trial Master File (TMF & eTMF) management solutions, is adopting Version 1 of the eTMF Exchange Mechanism Standard (eTMF-EMS) of the TMF Reference Model (TMF-RM) into PhlexTMF, the company’s eTMF technology. This marks a major advancement in efficient document interchange and will firmly solidify Phlexglobal’s position as a driving force in eTMF management for clinical trials.
UK: +44 (0) 1494 720420
US: +1 (484) 324-7921
Poland: +48 81 45 46 132
Germany: +49 89 23514741