HOW TO SURVIVE A TMF INSPECTION WHEN YOU'RE NOT PREPARED
You’ve just received an inspection notice, and the adrenaline is starting to kick in. When was the last completeness report? Quality review? Did the team finish the latest training on SOPs and the new eTMF system? Where to start?
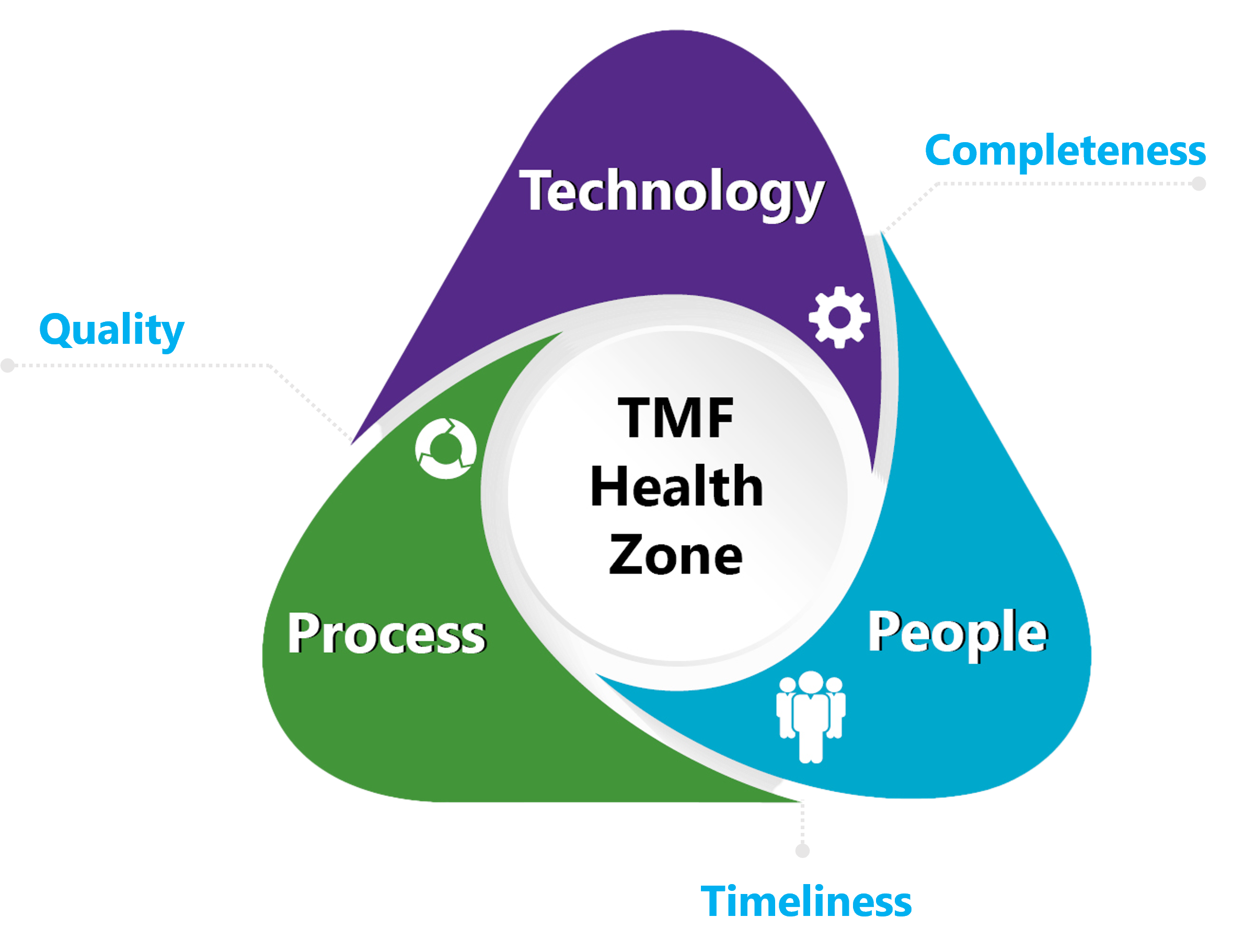
At a Glance
Phlexglobal’s Expertise Makes a Difference
Innovative Solutions
Reduces Inspection Risk
Offers Global Resources
Increase Output Speed
Brings Quality & Value
Better Value for Money
Phlexglobal Provides Unmatched TMF and Document Management Expert Services

TMF Quality Review
A thorough quality assessment of your TMF, giving you insight into its quality and completeness
TMF Heatmaps
Easily identify potential TMF problem areas and enable a risk-based remediation approach
Migrations & Imports
Help ensure you are meeting GCP standards and regulatory requirements for essential documents
Mergers & Aquisitions
Get a true picture of your acquired drug’s readiness for inspection or submission – and fix any problems


TMF Study Owners
Our TMF practitioners have successfully mastered the challenges you and your teams face every day
And Much More
Continue here to find out how to stay in the TMF Healthzone with expert, scalable services from the global TMF authority
Reduce TMF Misfiles with Next-Generation AI
Latest Insights
Check out our blogs, webinars, articles and more. Connect with our experts and stay up-to-date on all of the trends in the pharmaceutical, biotech and medical device industries.

Smart Steps to Managing Your TMF Audit Trail
The term audit trail can be daunting for companies, but this is a regulatory requirement. Simply put, when it comes to the trial master file (TMF), if an action is not recorded in the audit trail,

Lowering the TMF temperature through quality reviews
We are thrilled to announce Season 2 of our exciting webinar program, Summer Shorts: TMF Excellence Edition! Avoid boring summer reruns and attend these fresh, informative sessions where our experts

Five-step Game Plan for a TMF Close-out
Close-out of a clinical trial raises many questions about responsibility and management of the trial master file (TMF). According to ICH E6 (R2), final close-out can only occur when the monitor – or
.png)