
Few CROs offer compliant maintenance of the Trial Master File following study close closeout, usually returning the eTMF on unsecure and hard-to-track media such as USB drives or even printed out. These Trial Master Files are difficult to review and inspect, and force sponsors to keep their own study documents separate. Sponsors had limited options for compliance with regulatory requirements – until now.
The graphic below was inspired by an MHRA presentation from 2018 by Andy Fisher.
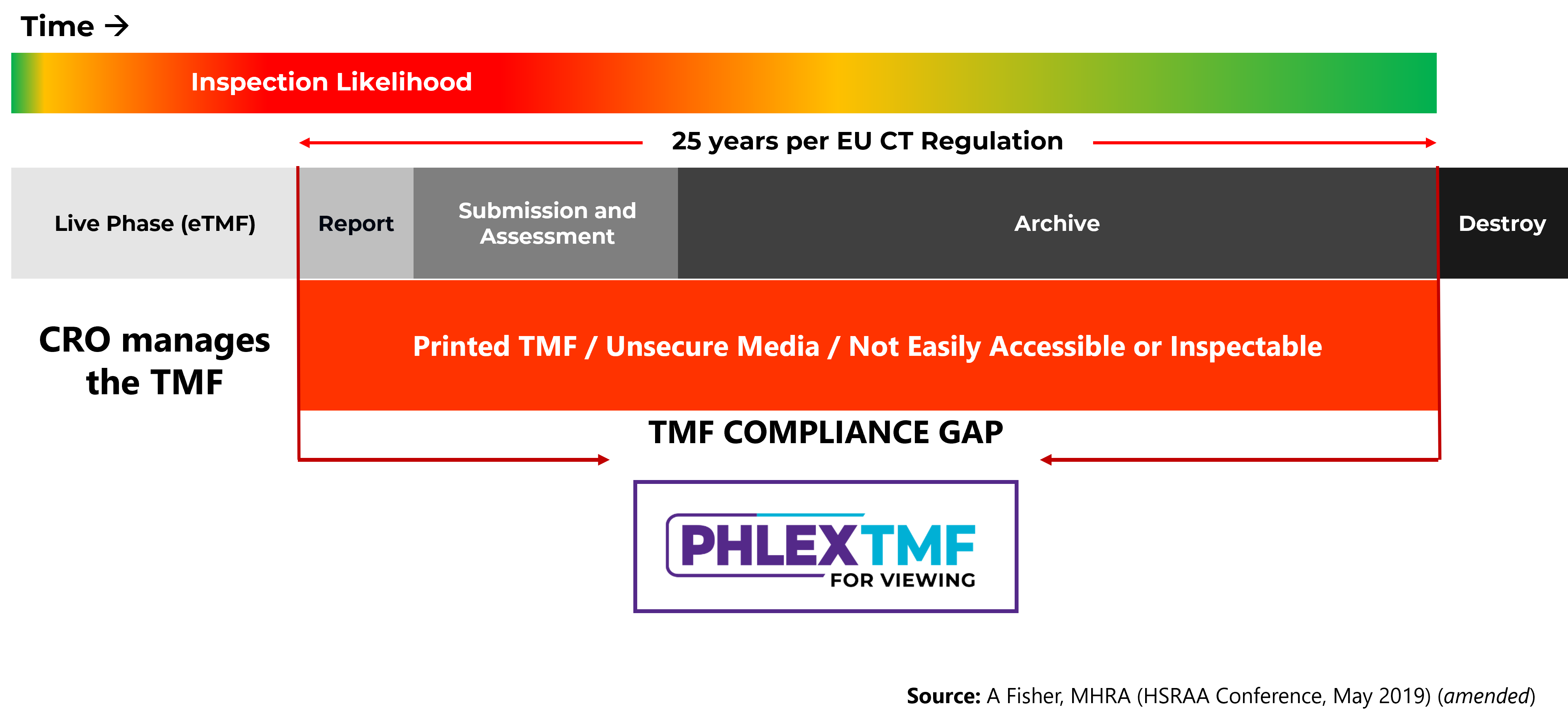
Note: Phlexglobal customers already have this capability as part of the PhlexTMF eTMF software.
Includes everything required to transfer eTMF documents, metadata, and audit trail into PhlexTMF for Viewing
Makes your TMF available for internal use and inspection in an intuitive user interface
Designed for ease of navigation and inspection prior to final archiving
Enables easy transition from Viewing Mode to long-term digital preservation
Powered by PhlexTMF advanced, purpose-built eTMF software
| Usual Industry Approach | PhlexTMF for Viewing Provides | |
| TMF is often returned to sponsor on unsecure and hard-to-track media such as USB drives. | Fully compliant, secure, cloud-based storage | |
|
Many documents still printed out - even if the CRO is using an eTMF |
Easy for sponsors to review at any time from anywhere | |
| Difficult for sponsors to review | Facilitates streamlined access for inspectors, which has become an expectation among regulatory agencies | |
| Sponsor documents are typically separate | Sponsor documents can be added directly to PhlexTMF for Viewing |

PhlexTMF for Viewing includes everything you need to successfully bring your TMF in house

The term audit trail can be daunting for companies, but this is a regulatory requirement. Simply put, when it comes to the trial master file (TMF), if an action is not recorded in the audit trail,

We are thrilled to announce Season 2 of our exciting webinar program, Summer Shorts: TMF Excellence Edition! Avoid boring summer reruns and attend these fresh, informative sessions where our experts

Close-out of a clinical trial raises many questions about responsibility and management of the trial master file (TMF). According to ICH E6 (R2), final close-out can only occur when the monitor – or
UK: +44 (0) 1494 720420
US: +1 (484) 324-7921
Poland: +48 81 45 46 132
Germany: +49 89 23514741