
Phlexglobal’s TMF Quality Review (sometimes called a “file review”) provides a line-by-line evaluation of your TMF, giving you detailed insight into its quality, completeness, and timeliness. Much more than just a series of TMF data reports, the customer-proven review is conducted by our expert TMF practitioners with deep understanding of current inspection standards at each major regulatory agency.

5 of the Top 10
Global Pharma
Companies
Are leveraging Phlexglobal's TMF Quality Review to reduce time, cost, and potential risks from M&A activity
Phlexglobal’s TMF Quality Review easily pinpoints key patterns and problem areas, further filtering the detailed review data to gain actionable insight for remediation. We can also able to take a functional service approach to the Quality Review, focusing on those areas where you don’t have internal expertise, or where you have already identified areas requiring deeper evaluation.
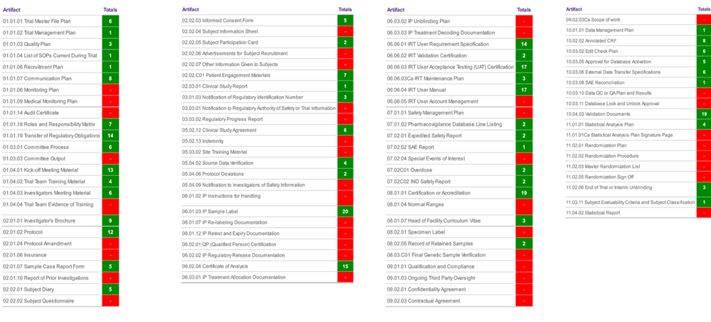
Detailed heatmaps are just one of the advanced tools utilized by Phlexglobal’s experts to help assess the quality of your TMF.


The term audit trail can be daunting for companies, but this is a regulatory requirement. Simply put, when it comes to the trial master file (TMF), if an action is not recorded in the audit trail,

We are thrilled to announce Season 2 of our exciting webinar program, Summer Shorts: TMF Excellence Edition! Avoid boring summer reruns and attend these fresh, informative sessions where our experts

Close-out of a clinical trial raises many questions about responsibility and management of the trial master file (TMF). According to ICH E6 (R2), final close-out can only occur when the monitor – or
UK: +44 (0) 1494 720420
US: +1 (484) 324-7921
Poland: +48 81 45 46 132
Germany: +49 89 23514741