Rewind the clock and take TMF back to basics: The paradigm of maintaining simplicity while embracing technology.
In 20 years, the Trial Master File (TMF) has moved from paper in dusty lever arch files, to electronic systems with placeholders, workflows, and artificial intelligence. The race for the most advanced eTMF is most certainly underway. However, in a world surrounded by technology it is easy to forget the basic principles of the TMF – the ‘what’ and the ‘who’ – as we focus so much on the ‘how’. We shouldn’t have those moments where we whisper quietly to our colleague, “it was so much easier when it was paper”.
Let’s start by pressing rewind…
My career in the Trial Master File started back in 2004. My first role was scanning vast quantities of TMF documents, to be indexed and uploaded in to an electronic TMF. By today’s standards it would be considered very primitive, and the Sponsor later upgraded to what is now one of the top eTMF systems in play; however, at the time, this was still pretty new technology. The other part of my role was spending time, more time than was healthy surrounded by dusty paper files, preparing documents for archiving, and shipping them off to be stored in an aircraft hangar somewhere in the middle of the countryside – probably, never to be seen again. However, if you take a moment to compare this with the TMF landscape of today, it’s clear to see just how far things have progressed in a relatively short period of time.
But, before we all get drawn down the technology rabbit hole, we should take a moment to remember the purpose of the TMF – that is to hold the essential TRIAL records in a MASTER repository, each having a specific FILE location. In practice it is nothing complex and bears an uncanny resemblance to any library – albeit an important one! What that TMF looks like in reality and how the stakeholders interact with it remains open to interpretation. Therefore, as subject matter experts we have a responsibility to help facilitate the management of these records in the best way possible.
…before pausing to ask THAT question!
So, was it? Was paper easier? Well, in many ways – yes, it was!
The process of filing clinical trial documents was very linear – documents were collected from the study team or site, they would be packed into a box or envelope and sent to whoever was responsible for filing them in the TMF (yes, another role I’ve done). QC checks were limited to what could be seen on paper – were signatures present, was everything legible, were all pages present – and then we filed it away into the respective folder while tracking it in Excel, because who doesn’t love another spreadsheet.
Importantly, it was a process that could be easily understood; it was ‘seen’, and we were dealing with physical documents. This concept was transferrable across organizations and departments, and that perhaps is where we find one of the key challenges of new and ever-developing technology.
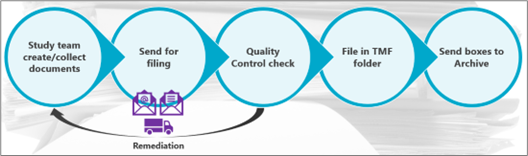
However, paper certainly came with flaws – there’s a reason we’re not still surrounded by paper! To really elevate the status of the TMF and keep up with regulatory expectations, it needed to be actively managed – rather than being a dusty library, it needed to be an interactive repository open to a wider but controlled audience; it needed to allow for better oversight – after all, it contains the dynamic story of the clinical trial.
Fast-forward to the realm of the eTMF
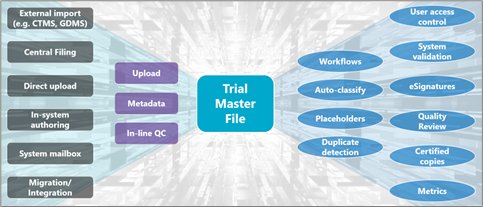
So, let’s bring ourselves back to the present day… Our linear process goes out the window and we are transported to a technology driven realm of electronic documents and records. We have numerous sources or methods of uploading, metadata expectations to classify documents, Quality Checks to ensure document content and metadata are accurate – and that is before we even start to explore other eTMF system requirements and functionalities from validation to eSignatures, placeholders, and metrics.
It can certainly feel that we’ve transitioned very quickly from one extreme to the other, and in an industry that is fundamentally built on the advancement of healthcare technology, it’s easy to think it’s not a bad thing. However, what happened to the middle ground? Let’s not forget, while eTMFs have developed significantly to give us best in class options, other file storage and sharing technologies have advanced too. We’re no longer limited to office-based servers and a CD or USB drive to share data, as cloud-based storage relying on off-site data centres are become the norm.
The battle of Organization and TMF maturity
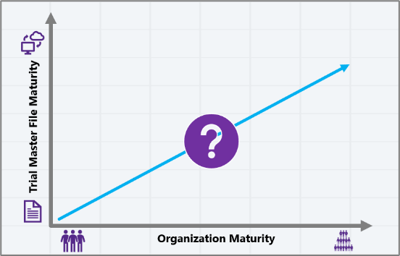
While the healthcare technology is advanced, the organizations in which it’s being developed take time to match the same level of maturity. A small biotech in the first stages of developing a novel drug, running Phase I clinical trials, may only just be giving serious thought to the Trial Master File – outsourcing 99% of activities to a CRO. However, a Sponsor with a few promising products may be realising that with a first submission and inspection somewhere in sight, the TMF suddenly needs a bit more attention and evidenced oversight!
In either situation we could expect to find an immature TMF model – maybe one or two individuals tasked with the responsibility of maintaining the trial documentation – maybe still partially held in paper at least for wet ink documents. At the other end of the spectrum, we have global Sponsor organizations with very well managed Trial Master Files taking full advantage of the features that well-established eTMFs like Veeva, Wingspan and PhlexTMF have to offer.
So how do we help organizations get from one extreme to the other without taking them down the technology rabbit-hole before they are ready as an organization, let alone a department?
How do we keep things simple?
How do we ensure the little fish have the tools to be as successful as the big fish?
Can we find a cost-effective solution that is not a purpose-built eTMF?
After all, a successful submission followed by an approved drug from any organization has the potential to do significant good.
Find your balance of power!
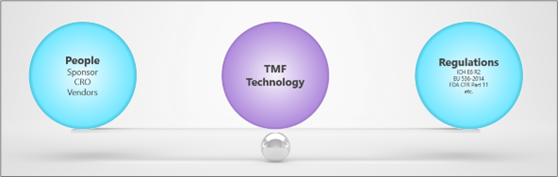
While we would like to see people at the centre, the reality is that when we’re looking at implementing technology, the balance really comes down to the needs of the TMF stakeholders, and the needs of regulations. These are the two factors that need to be in balance to achieve an efficient and compliant Trial Master File.
People are key to success, however it’s not always about having the ‘right’ people, but simply by understanding the organization and the stakeholders involved. Who will be using the TMF? Will it contain Sponsor documents only, or CROs documents too? How many people are involved in managing the TMF – 2 or 20? What is their level of experience?
When something is not going right with the TMF, it can come down to lack of understanding or appreciation of the people and the processes – not recognising the areas they struggle with, or trying to run before you can walk. A lot of the time it comes back to the organization maturity, and just because an ‘off the shelf’ TMF system can do something, doesn’t mean we need it to straight away.
Regulations are relatively static, and they don’t change significantly over time – but they can be challenging. It’s important that the crucial requirements are understood, especially when addressing an electronic Trial Master File. However, it’s also easy to over-complicate the requirements and try to implement more than you have the capacity to manage – and this is where it may be necessary to re-find balance. Take the examples below:
- Metadata – look at what the minimum requirements for efficient retrieval are – the fewer you implement, the less that need to be checked for accuracy.
- Security and access – consider the minimum number of roles you can utilize; why have 20 different user types, if you only need 3?
- Certified copy process – if the number of wet ink signatures are minimal and can be collected for archive separately – don’t try and implement one!
It sounds like common sense, but it is very easy to get caught up with industry trends and want to follow what other organizations are doing. There are also of course some elements of regulations that we can’t work around – validation of an electronic system, the need for CFR Part 11 compliant electronic e signatures – but the solutions for these are relatively straightforward.
In the quest for simplicity, take a moment to consider how well you balance the People with the Regulations, and whether that is resulting in the most efficient use of your TMF technology. After all, we are not necessarily striving for perfection, we’re looking for balance.
Building strong foundations
So how do we start in ensuring we are implementing simple, efficient, but compliant TMF Management?
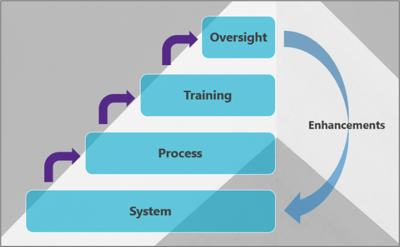
System – start with the right one for your organization; it is by no means a one-size-fits-all scenario, and the needs of the organization should play a key role in selection. I’ve worked across several TMF systems over the years, and I like each of them for different reasons and would recommend different ones to different people.
We are more frequently hearing the question of whether it’s possible to have a compliant electronic TMF without it being a purpose-built eTMF – for example utilizing Box or SharePoint. In principle I stand by that this answer should be ‘yes’ as the technology is sufficient to do so. The alternative is that you risk pushing smaller organizations out of the market altogether – which in healthcare could have real implications for patients.
I also want to take a moment to touch on AI here too, and the system enhancements that should make key processes more efficient – but the reality is that while they absolutely can, this doesn’t work for everyone. Do you have the resources to train, test and trust elements like auto-classify, duplication detection – they could certainly be considered game-changers, but the landscape for implementation has got to be right.
Process – make sure they are clearly defined and take note of what was easier when it was paper, along with what already works in your organization. Remember that a simple, linear process is easy to understand, so start with this and expand out.
Utilize industry resources too as they are there to help! The TMF Reference Model immediately gives you a structure to work from and is now recognized across organizations. It may have a reputation a bit like marmite, but even if people don’t ‘like’ it, they still recognise and know how to work with it!
Also ask yourself as you expand each process, is each new step adding value? Think about your risk-based approach – for example, which documents will you QC, what essential checks will you complete and what may not be necessary?
Training – do not underestimate the importance of training, especially for your end users. Making the TMF as easy and efficient as possible is often the crux of a healthy and compliant TMF. Make sure the training is delivered at the right time, with the right supporting resources, and with the opportunity for feedback that is actually taken on board.
Remember, it’s not just about how you get from A to Z, it’s about what that means in practice for the user – raise your game further and help them understand the why too!
Oversight – once you’ve got the foundations in place, only then should you start to focus on the oversight. Ask yourself, from the system and processes you’ve created, what do you want and need to measure? Don’t try and decide what you want to see, then make the everything else fit to that ideal. While I don’t want to delve too much into Metrics and KPIs (you’ll find that in another blog), Completeness, Quality and Timeliness remain the big three and can be done in a relatively simple fashion – even outside of a purpose-built eTMF. While this may require more manual manipulation, it could still be the most cost-effective option.
Again, we come back to balance and not perfection.
Our TMF maturity goal?
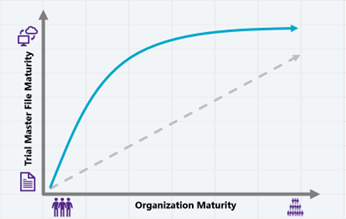
Revisiting the correlation between organization and TMF maturity – perhaps our goal needs to be to try and speed up the elevation in TMF maturity, but shifting focus away from the latest technology, and back towards people and simplicity.
The maturity of your TMF management should not be directly linked to the amount or complexity of TMF technology you can afford employ. If the regulators recognise that perfection is rarely achievable, then TMF perfection should not be the goal.
Instead, let’s mature by focussing on compliance and enhancing the understanding of the changing regulations. Not what these mean in an ‘ideal’ world, but what they mean in the ‘real world’, and how these can be balanced with organizational need. We can take the lessons we learnt from paper TMFs and transition them to the realm of technology – after all, drugs still got approved before there was an electronic record in sight…
5 Key Principles for a Healthy TMF
To round up, I want to leave you with 5 key principles that can set you on the way to healthy and happy TMF Management:
- Start simple, end simple – how you chose to set up your TMF at the start will have a direct impact on how you come to archive at the end. The fewer systems you can have your TMF records spread between, the less you will need to consolidate at the end of the trial.
- Minimize processes – be thoughtful about your processes and relationships – make them lean and make them efficient – this will drive the overall level of quality.
- Invest in the people – investing time and understanding in your TMF stakeholders will pay off in the long run.
- Learn the regulations – do your own research and understand how they apply to you, don’t always rely on subjective opinion.
- Enhance before advance – make sure you’re getting the full potential out of your existing system before taking the step to advance to a new one.
Take these principles on board and hopefully you will be left with a smile…
The contents of this blog are solely the opinion of the author and do not represent the opinions of PharmaLex GmbH or its parent Cencora Inc. PharmaLex and Cencora strongly encourage readers to review the references provided with this blog and all available information related to the topics mentioned herein and to rely on their own experience and expertise in making decisions related thereto.
.png?width=300&name=PharmaLex_RGB%20(1).png)

