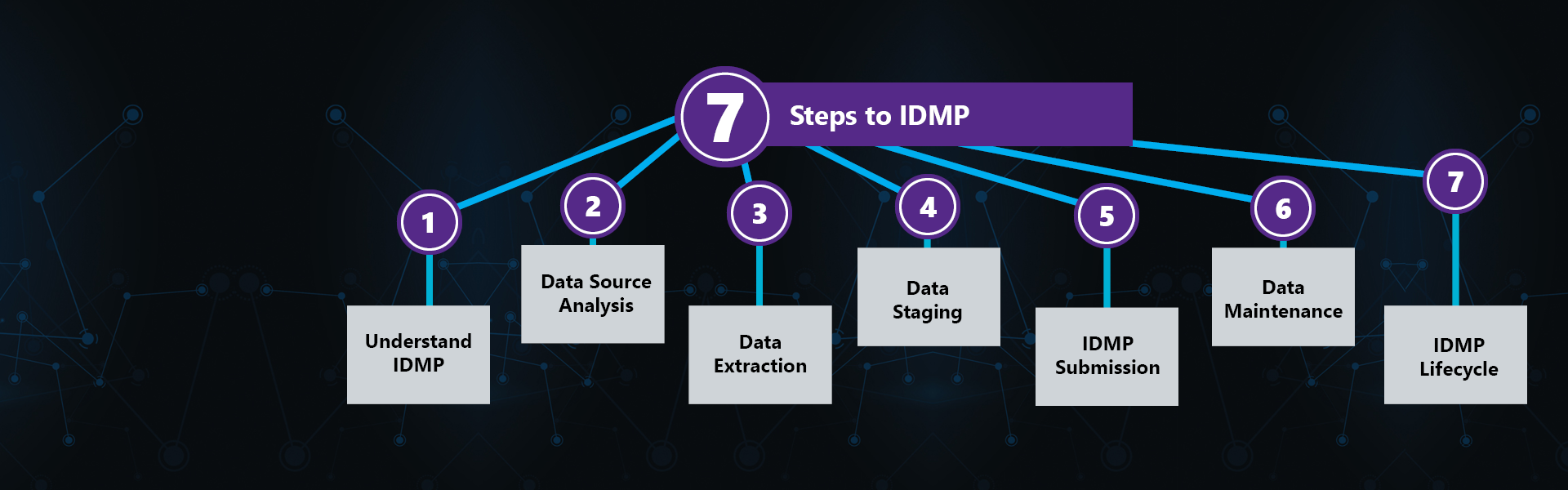
IDMP (Identification of Medicinal Products) refers to a set of 5 ISO standards - ISO 11615, ISO 11616, ISO 11238, ISO 11239 and ISO 11240 – designed to standardize the structure and terminology used to describe marketed medicinal products. IDMP’s primary goal is to enhance patient safety by improving the speed, accuracy, consistency, and sharing of adverse events and safety signal reporting globally. As a far-reaching, complex set of standards, IDMP promises to significantly impact regulatory affairs and operations, pharmacovigilance, CDC, marketing, IT and more for Marketing Authorization Holders. Most organizations will require at least 12-18 months to fully prepare for IDMP compliance.
While IDMP presents a challenge, forward-thinking organizations are looking at IDMP as an opportunity to dramatically improve data processes across the regulatory lifecycle by utilizing advanced technologies such as AI-driven automation.
Join us for this webinar to see how you can build an agile foundation that can quickly adapt to new regulatory and business requirements in a consistent way across the organization, enhancing safety while reducing time, cost, effort and organizational risk.
 |
Jim Nichols Chief Product Officer |